

INTRODUCTION
RhD Education aims to help obstetricians-gynecologists, midwives, doctors interested in the problems of blood group immunizations and women, to keep up to date on the problems created by the incompatibility of different blood groups between parents which then have repercussions on the development, the well-being and the health of their future child. In particular, even though the problem of Rh factor incompatibility has been known for almost a hundred years and prevention, implemented through immunoprophylaxis, was introduced more than fifty years ago, this problem still affects around 50 % of pregnancies worldwide which are characterized by consequent perinatal mortality, morbidity and/ or serious post-natal problems.
The recent guidelines promoted by FIGO (the International Federation of Gynecology and Obstetrics) and ICM (the International Confederation of Midwives) published at the end of 2021 confirm that the main strategies are to return to focusing the attention of doctors and women on this problem, introducing, where it does not yet exist, the routine typing of blood groups in women of reproductive age or at the beginning of pregnancy, investing in the free administration of immunoglobulins as a public health priority in all countries of the world. And these are also the objectives that a Consortium, created in New York at Columbia University in 2018 and called WIRhE (Worldwide Initiative for Rh disease Eradication), has set itself by creating an International Academy of Advisors who not only contribute to this website but they will help keeping Rh Educational up to date and interesting. These advisors also represent, in many regions of the world, ambassadors to implement the above strategies. The WIRhE Academy guarantees the quality and scientific nature of the contents of RhD Education written by experts and based on the most recent scientific literature.
WHAT TO KNOW ABOUT HDFN
How do antibodies from the mother attack the fetus in HDFN?
Hemolytic Disease of the Fetus and Newborn (HDFN) is a disorder caused by the attack mediated by the sensitized maternal immune system through specific antibodies targeted to blood group antigens on the surface of fetal or newborn red blood cells (RBC), lacking on the surface of maternal RBCs. HDFN can produce pathological consequences ranging from hemolytic anemia (mild to severe) and Hydrops fetalis in the fetus and severe neonatal hyperbilirubinemia and kernicterus in the newborn, conditions that can have significant morbidity and mortality.
- How does HDFN relate to the Rh factor?
- Epidemiology
- How can Rh hemolytic disease be prevented?
- Types of HDFN
- AB0 incompatibility
- Alloimunization to fetus or newborn RBCs antigens
- Rh System
- Which antigen is responsible for the most severe cases of HDFN?
- What is the pathophysiology of HDN?
How does HDFN relate to the Rh factor?
The most common antigen involved in this mechanism of alloimmunization due to antigenic incompatibility is the Rhesus D (RhD) antigen, but an ever-increasing number of different antigens potentially involved in the pathogenesis of the disorder has been found since the first description of the disease by Dr. Louis K. Diamond in 1932. when he wrote about erythroblastosis fetalis in the newborn based on peripheral smears. The International Society of Blood Transfusion Working Party for Red Cell Immunogenetics and Blood Group Terminology (ISBT WP) recognizes 43 blood group systems containing 345 red-cell antigens currently.
Epidemiology
Rh incompatibility has a global distribution that varies in different geographical areas by race, ethnicity, and risk factors. The Rh-negative blood type is most predominant in white races (15%) compared to African Americans (5% to 8%) or Asians and Native Americans (1% to 2%). This means that a Rh-negative white woman has an 85% chance of mating with a Rh-positive man.
This frequently occurring Rh incompatibility increases the risk of HDFN with any fetomaternal hemorrhage (FMH) event. It should be underlined that an amount of only 0.1 mL of fetal blood entering maternal circulation is sufficient to cause alloimmunization.
How can Rh hemolytic disease be prevented?
Owing to the prevention, the early detection and the management of this disease made possible by the improvements in pathophysiology comprehension and in pharmacological resources, the incidence and prevalence of HDFN have exponentially decreased in the past 50 years.
Rhesus D-negative (RhD) immunoprophylaxis was first introduced in 1968, which allowed a drop in the incidence of HDFN from 1% of all newborns worldwide (with almost 50% mortality) to 0.5%. The incidence of HDFN decreased even further to 0.1% with the administration of antepartum Rh(D) immunoprophylaxis.
The combination of antenatal and post-partum immunoprophylaxis is almost 99% effective at preventing maternal sensitization to Rh(D).
Therefore, most current guidelines prepared by various associations of healthcare professionals involved in the prevention and in the management of HDFN recommend immunoprophylaxis with IgG anti-Rh(D) to every non-sensitized Rh (D)-negative woman, with different timing depending on the situation:
(1) at 28 weeks of gestation during each pregnancy
(2) immediately after delivery of every Rh(D)-positive neonate
(3) in the context of any other event that could expose the mother to the Rh(D) antigen (e.g., abortion, miscarriage, abdominal trauma)
However, on the basis of the annual number of doses of anti-Rh(D) produced and provided worldwide, it has been estimated that almost 50% of the women around the world who require this type of immunoprophylaxis do not receive it, presumably due to a lack of awareness, availability, and/or affordability, thereby putting hundreds of thousands of fetuses and neonates at risk for Rh disease each year.
The gap between IgG anti-Rh(D) supply and demand is larger in low-income countries, such as Asia South and Sub-Saharan Super Regions, Asia East, S. East and Pacific countries, Eastern Europe/Central Asia countries, Latin America and Caribbean countries, North Africa/Middle East countries, but also in high income countries, immunoprophylaxis for maternal Rh(D) sensitization is below the optimum threshold required to guarantee complete prevention.
More efforts are needed to overcome this yet diffused situation, starting from a wider awareness of the disease and of the means to prevent and treat it.
This website has this specific mission.
Types of HDFN
Hemolytic disease of the fetus and newborn (HDFN), also known as erythtoblastosis fetalis, is an immune-mediated disorder in which maternal antibodies attack fetal or newborn red blood cells (RBCs) due to antigen incompatibilities, with hemolysis in the fetal or neonatal blood circulation.
HDFN can cause significant morbidity and mortality, especially in limited healthcare resource settings.
The mechanisms through which maternal antibodies are developed and attack fetal or newborn RBC antigens are mainly represented by ABO incompatibility and alloimmunization due to maternal sensitization to fetal or newborn RBCs surface antigens by transfusion and fetomaternal hemorrhage (FMH) during pregnancy or at the time of delivery.
Various types of HDFN are classified by which alloantigen provokes the response. The types include ABO, anti-RhD, anti-RhE, anti-Rhc, anti-Rhe, anti-RhC, multi-antigen combinations, and anti-Kell.
AB0 incompatibility
ABO incompatibility is a congenital mismatch between maternal and fetal blood types, that occurs in 15 to 20% of pregnancies. The incompatibility between the mother and the fetus blood antigens of the ABO system is the leading cause of neonatal jaundice and the most common form of HDFN, but clinical severe HDFN will develop only in about 1% of cases of blood types mismatch.
It is thought that the generally milder disease caused by ABO incompatibility is due to the expression of A and B antigens by placenta and other tissues and therefore these antigens may associate with maternal alloantibodies to some extent. Furthermore, the expression of A and B blood group antigens on fetal red cells is not fully developed and so there are fewer antigenic sites on the fetal RBCs surface for the antibodies to bind with.
It must be remembered that individuals naturally begin to make A and/or B antibodies to the antigens that they do not possess at approximately 3–6 months of age. As a result, ABO HDFN can occur with the first pregnancy without any prior foreign antigen exposure, unlike other types of HDFN, is observed almost exclusively in mothers with blood type O and is more common with A fetal blood type.
Mothers with O blood type have naturally occurred immunoglobulins of G class (IgG) against A or B blood types, capable to cross the placenta. During pregnancy, the formation of anti-A, anti-B and anti-A, B titles of the IgG class can be strongly increased.
Both A and B groups patients also develop the respective antibodies to the antigens they do not possess, however they are not normally at risk of HDFN as these antibodies are almost always of IgM class and thus cannot cause fetal or neonatal disease because they do not cross the placenta.
In very rare instances, ABO incompatibility has been implicated as the cause of severe fetal anemia and perinatal morbidity. There is a striking, and yet not understood, difference in the incidence of ABO-mediated HDFN between populations. The incidence is around 0.3–0.8% in the Caucasian population, versus 3– 5% in Black or Asian populations, also with a more severe clinical course.
Alloimunization to fetus or newborn RBCs antigens
The mechanism most involved in HDFN is the alloimmunization due to the development of maternal antibodies targeted to an antigen present on the fetal RBC surface, when fetal RBCs enter the maternal blood circulation. The production of maternal antibodies against fetal RBCs antigens is elicited by previous transfusions and FMH.
The alloimmunization rate in the general population varies from 2% to 7%, with an increased likelihood in transfused patients. In pregnancy the rate is estimated to be in the range 0.4% to 1.2%.
After blood transfusion, 1% to 2% of people develop blood group antibodies; most are of little consequence, however some can induce a severe form of HFDN.
Alloimmunization due to FMH is an acquired immune-mediated mechanism that typically affects subsequent pregnancies rather than the pregnancy in which the FMH happens.
It has been shown that 75% of women have a FMH during pregnancy or at the time of delivery. In about 50% of these women, the FMH does not exceed 0.1 mL of fetal blood. Less than 1% of women have more than 5 mL, and less than 0.25% have more than 30 mL of fetal blood in their circulations. Some obstetric conditions increase the risk of FMH including antepartum bleeding, toxemia of pregnancy, external version, cesarean section, and manual removal of the placenta.
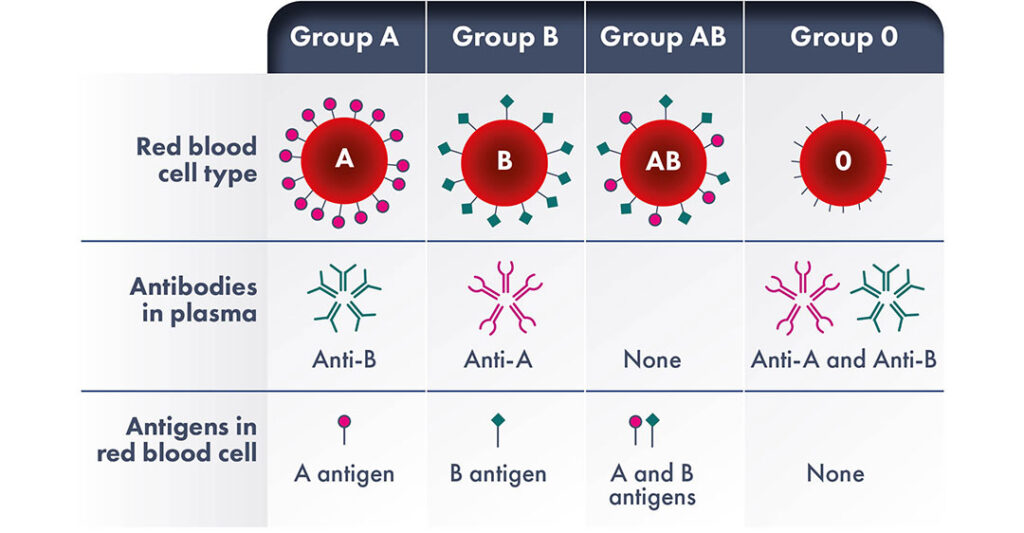
Antigens and immunization
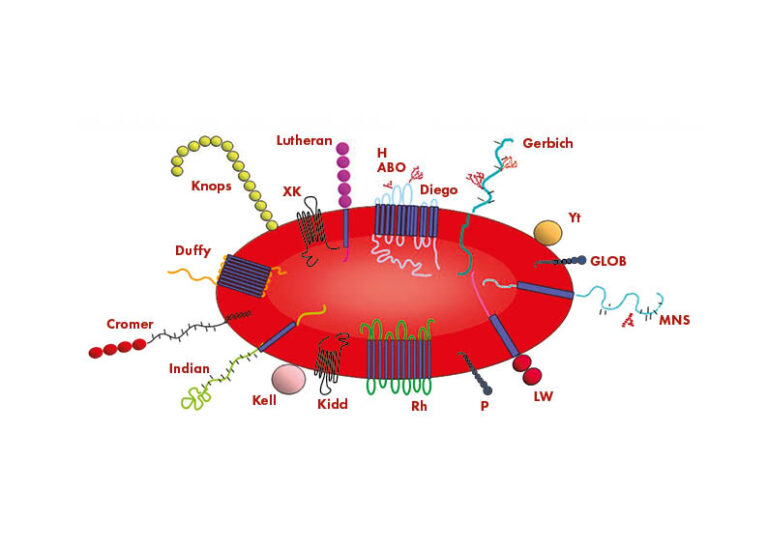
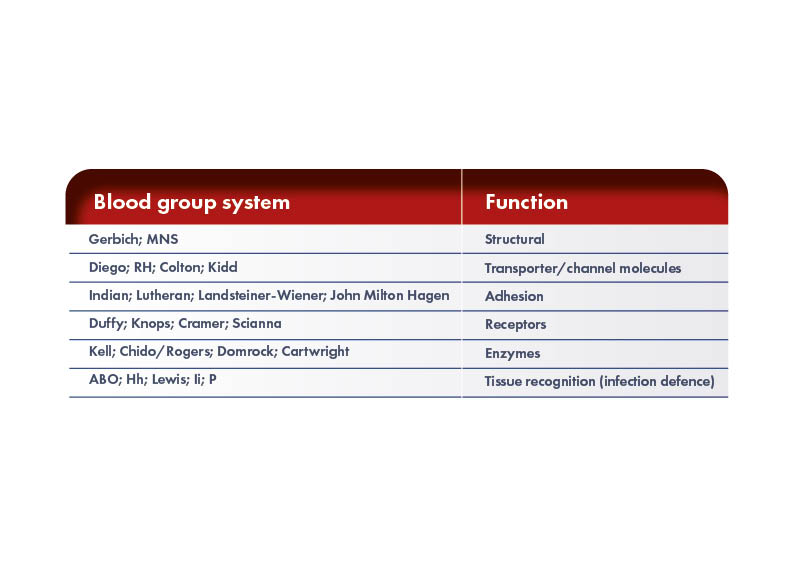
Individuals have different antigens on the surface of their RBCs. Some of these antigens are responsible for the development of the immune response, leading to HDFN, whilst many are not. The International Society of Blood Transfusion Working Party for Red Cell Immunogenetics and Blood Group Terminology (ISTB WP) recognizes 43 blood group systems containing 345 red-cell antigens. However, new antigens are constantly discovered and thus the knowledge of potential antigen:antibody complexes continues to increase. The RBC antigens in the different blood group systems are proteins or glycolipids and can have different functions.
Rh System
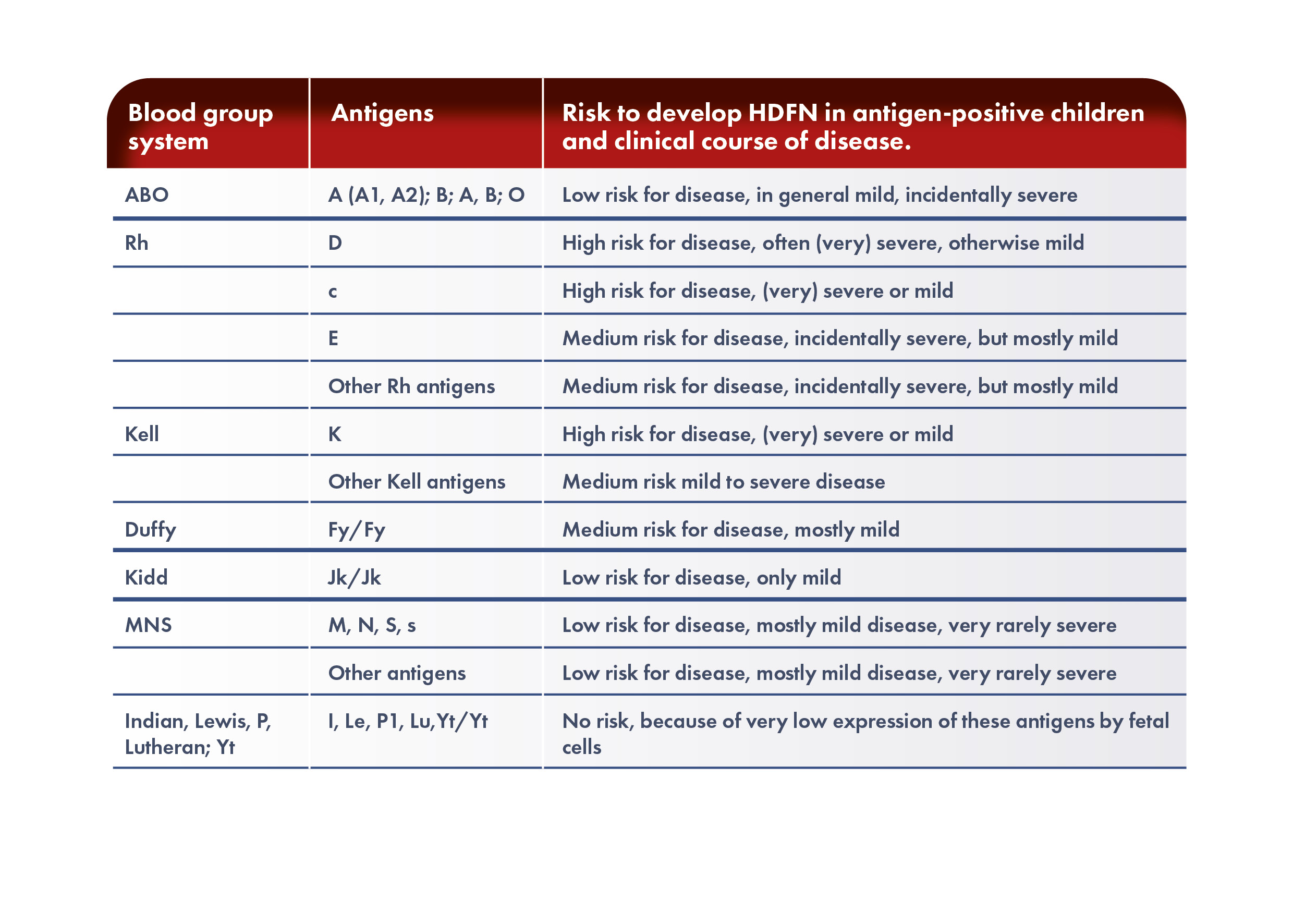
The different RBCs antigens, within and among the various blood group systems, have different impact on the antibodies production, but only a minor proportion on these antigens cause red-cell incompatibilities clinically relevant. The most frequently surface antigen of RBCs involved in severe form of HDFN is the Rhesus factor, discovered in the 1940s in Rhesus monkeys.
The Rh blood group system is the is the second clinically most important after the ABO system. As far as we know to date, the Rh system is made up of 55 surface inherited antigens of which the main are the D, C, c, E and e antigens that are codified by two highly homologous genes, RHD and RHCE.
The most immunogenic antigen is the D antigen (RhD). A person is considered as Rh-positive (RhD+) or Rh-negative (RhD-) based on the presence or absence of the RhD antigen on RBCs’ surface. An antigen d has never been proved to exist. The antigens are inherited in two sets of three, one set from each parent. The most common sets are CDe(R1), c(d)e(r), and cDE(R2)
Slightly fewer than 50% of Rh-positive people are homozygous for D (i.e., they have inherited a set of antigens containing D from both sets of parents). If a positive mate of an Rh-negative woman is homozygous, all of his children will be Rh-positive whilst if he is heterozygous, the fetus will be Rh- negative or Rh-positive with equal chance. Only Rh-positive fetuses can cause Rh immunization and the production of anti-D antibodies in RhD-negative women determines erythroblastosis fetalis in RhD-positive fetuses. The D antigen is already present from the sixth/seventh week of gestation and that 0.1 mL of fetal red blood cells D + can alloimmunize an Rh-negative mother. The RhD-positivity varies among the ethnicities: it is reported in 85% of Caucasians, 95% in sub-Saharan Africans, and more than 99.5% in eastern Asians.
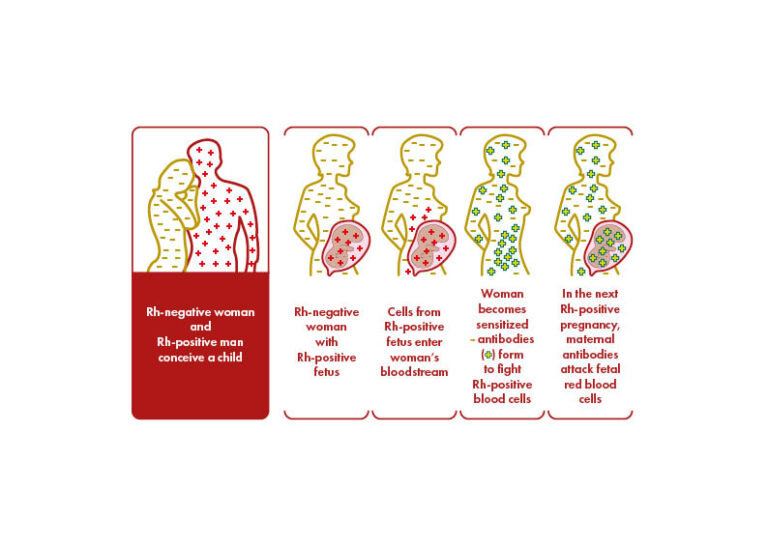
Which antigen is responsible for the most severe cases of HDFN?
The RhD antigen is the most immunogenic among the antigens of Rh system and anti-D antibody is correlated with the highest risk of fetal mortality and morbidity. Rhesus antigens other than RhD including c, C, E, and e are often implicated, and can produce severe hemolysis. However, also antigens from Kell, Duffy, MNS, and Kidd blood group systems are known to cause severe form of HDFN. Understanding the different red-cell incompatibilities and antibody production that cause HDFN is important to predict, prevent, monitor and manage pregnancies and neonates at risk of the effects of fetal and neonatal hemolytic disease.
What is the pathophysiology of HDFN?
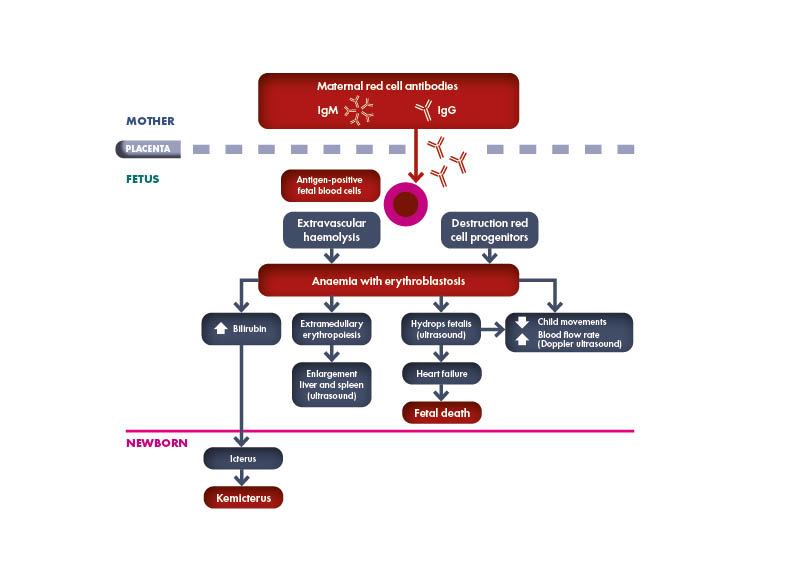
HDFN occurs when the fetus or neonate inherits a RBC antigen from the father that is not present on the mother’s red blood cells. The non-self RBC antigen elicit the maternal production of specific antibody. Maternal IgG antibodies travel across the placenta into the fetal blood stream, bind to the foreign antigen and cause hemolysis and possible anemia. Fetal anemia induces compensatory erythropoiesis, nonetheless this mechanism may be insufficient. Compensation for the anemia results in a hyperdynamic circulation in the fetus causing cardiomegaly and hepatic failure. Liver failure causes decreased protein levels and a reduction in oncotic pressure in the circulation and heart failure causes an increase in venous pressure; the combination of which leads to generalized edema and ascites, known as fetal hydrops, which has a high perinatal mortality rate.
Hemolysis of the fetal red cells results in raised bilirubin levels. Since bilirubin passes the placenta, excess of bilirubin is cleared via the maternal circulation during pregnancy. After birth, the hemolytic process continues, but the relatively immature liver of the neonate cannot sufficiently conjugate the excess of bilirubin. This may result in severe hyperbilirubinemia and even in irreversible damage to the central nervous system, a condition known as kernicterus. This condition is characterized by bilirubin deposition in the basal ganglia and brain stem nuclei and is correlated with long-term morbidity such as severe form of athetoid cerebral palsy, hearing problems and psychomotor handicaps.
ANTENATAL DIAGNOSIS
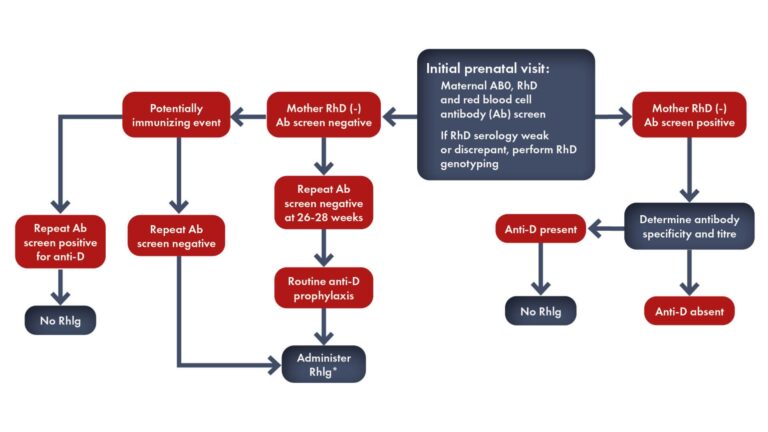
Rh-disease is usually diagnosed during the routine prenatal screening. Predicting the fetal RhD status by non-invasive antenatal screening for the fetal RhD gene (RHD) can guide targeted use of antenatal anti-D prophylaxis.
What is HDFN in pregnancy?
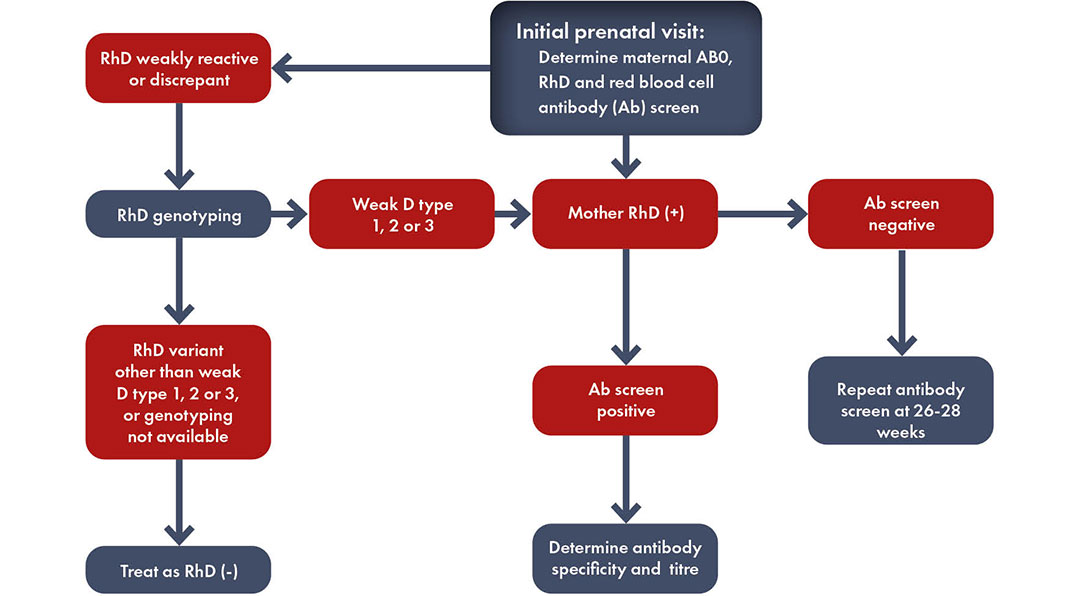
Every woman in pregnancy should be tested for blood grouping determination and antibody screening to identify the Rh status and the presence of an immunization that posese the fetus at risk of hemolytic disease of fetus and newborn (HFDN) also known as erythroblastosis fetalis.
According to the American Academy of Pediatrics (AAP), “if a mother has not had prenatal blood grouping or is Rh- negative, a direct antibody test (or Coombs’ test), blood type, and an Rh (D) type on the infant’s (cord) blood are strongly recommended.”
Blood group and detection of irregular RBC antibodies checked at week 28th of pregnancy. The Rh and ABO statuses of the father of the fetus of a Rh-negative pregnant woman should be determined. If he is heterozygous, the risk of Rh immunization is halved. If the ABO and Rh genotype of the father are known, the risk of Rh immunization can be estimated.
It is important to retest regularly a Rh-negative pregnant woman who has a Rh-positive mate. Further testing should be carried out at weeks 18 to 20 of pregnancy and every 4 weeks thereafter considering that an initial immune response rarely occurs before the week 20 of gestation.

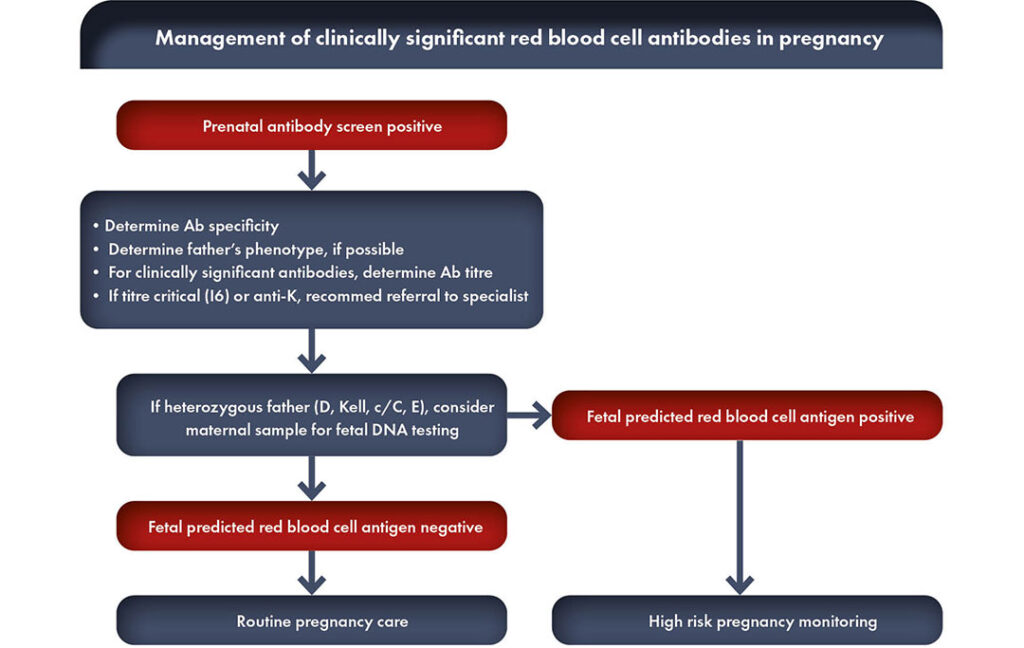
What are clinically significant red cell alloantibodies?
When the presence or development of red cell alloantibodies is not detected during pregnancy, the only, nonspecific, and unpredictable clinical features of HDFN during pregnancy are a decrease of fetal movements or sudden fetal death.
When the presence of maternal anti-Rh antibodies is identified by screening, the pregnant woman must be monitored by repeated laboratory tests (antibody titers, antibody quantitation, antibody-dependent cellular cytotoxicity or ADCC). If these serum exams are above a certain cut-off level, it is necessary to carry out clinical investigations in specialized centers such as Echo-Doppler sonography to timely detect severe HDFN requiring fetal therapy or delivery with neonatal treatment.
Thus, a screening program aims to identify those pregnancies in which intrauterine treatment of the fetus is needed and/or delivery should be induced to lower the risk of development of severe hemolytic disease. Without a screening program, treatment may be delayed.
What laboratory tests are involved in diagnosis of HDFN during pregnancy?
Since the fetus can become severely anemic from the 16th week of gestation onwards, non-invasive fetal blood group typing with cell-free fetal (cff) DNA from maternal plasma should be performed before 14–15 weeks for the screening of non-immunized D-negative women.
Because cffDNA represents only a fraction of the total cell-free DNA in the maternal plasma, highly efficient DNA extraction and sensitive detection technology, currently with quantitative PCR, is required.
Other methods applied to fetal RHD typing involve single-base extension, followed by demonstration of the specific products by GeneScan or mass spectrometer.
Recently, a chip-based digital PCR for fetal blood group typing has been described. This method is called droplet digital PCR (ddPCR). In a ddPCR each droplet contains only a single copy of the maternal or fetal gene, and the presence of a fetal copy will therefore always result in a strongly positive signal that can easily be discriminated from droplets containing maternal DNA copies.
Confirming the presence of fetal DNA with other paternally inherited DNA sequences (e.g. SRY) or a universal fetal DNA marker (e.g. methylated RASSF1a) is used by some laboratories to report conclusive results in one single assay. It can be concluded that non-invasive fetal genotyping can reliably be used to target laboratory and clinical monitoring to cases at risk for the development of severe HDFN.
Degree of severity of hemolytic disease
In immunized Rh-negative women it is of utmost importance determine the degree of severity of erythroblastosis (if any) in the fetus.
Six parameters can help to assess the degree of severity of hemolytic disease:
1) history of preceding disease
2) maternal antibody titers
3) cell-mediated maternal antibody functional assays
4) amniotic fluid spectrophotometric measurements
5) ultrasonographic assessment
6) direct fetal blood sampling.
The definition of severity is as follows:
- ‘Very severe HDFN’ refers to cases of intrauterine death and cases treated by intrauterine transfusions (IUTs) and/or exchange transfusion (ET) after birth.
- ‘Severe HDFN’ also includes cases treated with top-up transfusions and cases in which preterm induction of labor is needed.
- A ‘mild course’ is defined as cases without a need of pre- term induction of labor and in whom treatment with phototherapy suffices.
Hazards of amniocentesis and induction of early delivery
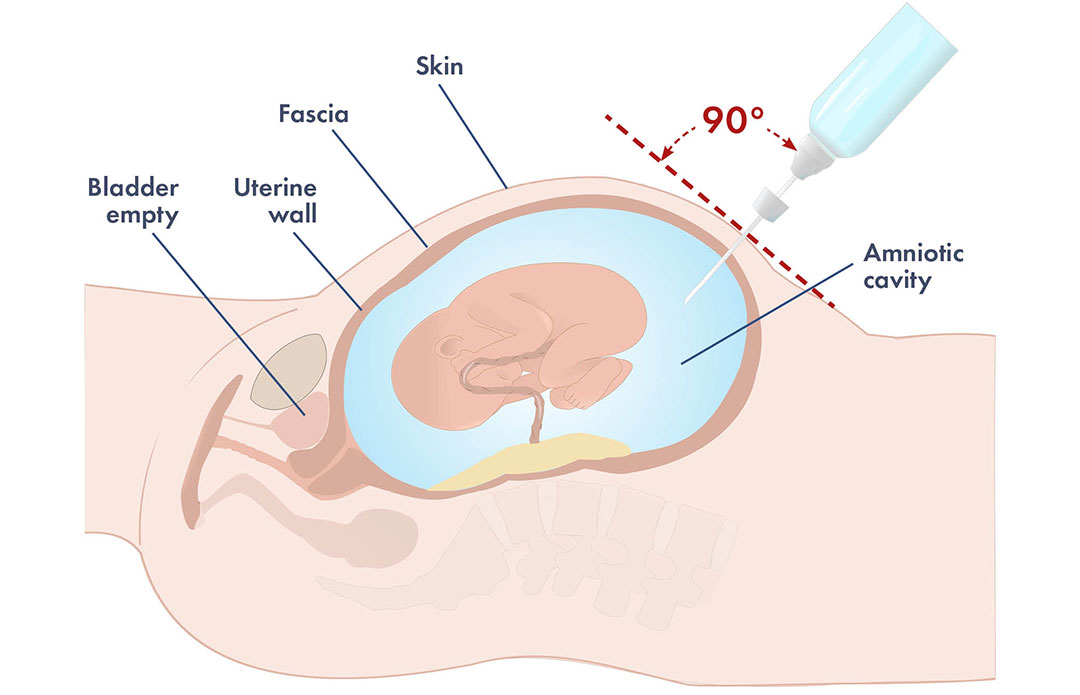
It must be kept in mind that owing to the always present risk of fetomaternal hemorrhage (FMH), with consequent increasing antibody titers and increasing severity of hemolysis, amniocentesis should be carried out only after careful ultrasound placental localization. Amniocentesis carried out without ultrasound guidance is associated with a 10% – 11% risk of FMH and increased severity of erythroblastosis. However, even if ultrasound-guided, amniocentesis, being an invasive maneuver, can determine FMH. Thus, it should be undertaken only if the history or antibody titer indicates that the fetus is at risk of developing hydrops and dying. Irrespective of antibody titer, amniocentesis should be undertaken at week 18 to 19 of gestation in case of history of previous stillbirth or if the previous newborn required ET after birth. In the absence of such history, amniocentesis should be performed only if there is an antibody titer indicating a consistent risk of hydrops.
Only the fetus who will be hydropic before the week 34 of gestation should undergo fetal blood transfusions, and only these fetuses and those who will otherwise become hydropic between weeks 34 of gestation and term should be exposed to the hazards of induced early delivery. The risk of death linked to fetal transfusion trauma (intraperitoneal) is 10 – 15% at weeks 22 to 23 of a pregnancy and falls to 3- 5% after week 28. In the premature erythroblastosis infant with a severe disease, the risk of death is still nowadays significant at week 32 of gestation.
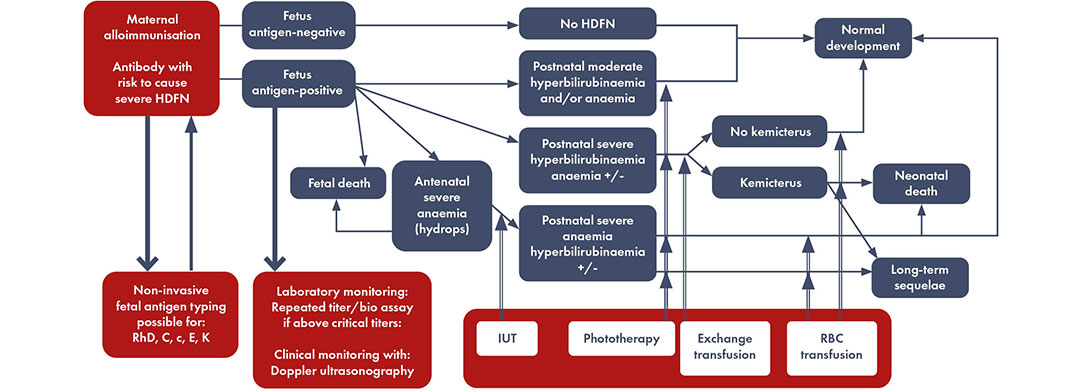
Disease model maternal alloimmunization leading to hemolytic disease of the fetus and newborn and laboratory testing and eventual clinical testing to discriminate high-risk cases. Modified by Haas M et al, 2015.
*If non-invasive fetal antigen typing is not available, pregnancies should be monitored as if the fetus is antigen positive when the biological father is homozygous or heterozygous positive for the respective antigen. Pregnancies can be monitored as if the fetus is antigen negative upon a negative non-invasive fetal antigen typing result, or when the biological father is typed with certainty as homozygous negative for the respective antigen
POSTNATAL EVALUATION
Many women have little or no detectable fetal blood in their circulation during pregnancy, and then only in small volumes (<0.1 mL) that may increase slightly in the third trimester. After delivery, the incidence and volume of FMH is usually greater and this explains why more women are D-immunized after delivery than during pregnancy.
Thus, it is extremely relevant that all D- negative women be screened after delivery for FMH; in case of a positive result the volume of fetomaternal hemorrhage should be quantified. High volumes of FMH can lead to fetal death or severe neonatal anemia requiring prompt transfusion.
D-negative women need to receive an adequate dose of anti-D immunoglobulin (anti-D Ig) to destroy all the fetal RBC in their circulation so to prevent D-immunization, which poses risks of HDFN in future D-positive pregnancies.
What are the findings of hemolytic disease of the fetus and newborn assessment?
Hemolytic disease of the fetus and newborn should be considered in the differential diagnosis of newborns with jaundice/hyperbilirubinemia and certainly in the case of neonatal anemia.
At birth, the connection to the maternal circulation is severed, because of the immature development of the metabolic pathway to break down bilirubin in the neonatal liver, the risk of neonatal hyperbilirubinemia increases significantly. The management of hyperbilirubinemia is critical in the neonatal period because of the risk for bilirubin-induced encephalopathy.
For newborns with known HDFN, close observation of bilirubin levels and hemoglobin is mandatory to determine whether neonatal ex- change transfusion is needed to wash out bilirubin and maternal antibody, and/or if transfusions are indicated to support oxygen carrying capacity to the tissues. Infants must be carefully monitored for clinical signs of ongoing anemia, whichis most likely manifested by poor feeding, the most aerobic activity for neonates. They may also have increased sleep as anemia worsens.
In ongoing anemia, the reticulocyte production from the fetal bone marrow may be decreased, and other cells lines, such as neutrophils can be affected. Weekly monitoring of reticulocytes and hematocrit will help to guide decision making about transfusion and provide reassurance when the marrow is recovering.
LIBRARY
It was in 1968 that anti-Rh(D) immunoglobulin was approved to prevent the onset of sensitization to the Rh(D) blood group antigen after delivery in Rh-negative women.
Afterwards, anti-Rh(D) prophylaxis was extended to include Rh-negative pregnant women, cases of miscarriage, ectopic pregnancy, amniocentesis, bleeding or abdominal trauma during pregnancy, and/or external cephalic version for breech presentation.
Even if this approach is highly effective to prevent Rh-disease, recent data have shown that, in approximately 50% of eligible cases world- wide, anti-Rh(D) immunoglobulin is not administered.
Insufficient supply, cost considerations, ignorance, lack of access to the immunizing product and use of products that have not been tested for therapeutic efficacy are the main reasons for this unsatisfactory preemptive coverage. It must be underlined that Rh disease is still cause of more than 160 000 perinatal deaths and 100 000 cases of disability annually. These worrisome data represent only a 50% reduction relative to the era before immunoglobulin administration, a situation that considering the high burden of a preventable disease should be considered unacceptable.
The International Federation of Gynecology and Obstetrics (FIGO) and the International Confederation of Midwives (IMC) have published in 2021 a joint guideline paper on Rh-disease prevention by immunoprophylaxis, that will be briefly summarized in this section.
FIGO & IMC
The International Federation of Gynecology and Obstetrics (FIGO) and the International Confederation of Midwives (IMC) have published in 2021 a joint guideline paper on Rh-disease prevention by immunoprophylaxis, that will be briefly summarized in the following sections.
Blood Group and Rh(D) Typing
A priori knowledge of the maternal Rh status is mandatory to prevent Rh sensitization. The Rh(D) typing should preferably be performed in the first trimester of pregnancy, because indications for anti-Rh(D) immunoprophylaxis may arise early during gestation.
Postpartum Anti-Rh(D) Immunoglobulin Administration
Rh(D) sensitization occurs in 16% of pregnancies of Rh(D)-negative women and Rh(D) immunoglobulin administration reduces this risk to 1.5%. Thus, this intervention should represent the highest priority in countries/regions where prophylaxis is currently inadequate or not provided. A dose of 1,500 IU (or, equivalently, 300 μg) of anti-Rh(D) should be administered intramuscularly within 72 hours after delivery in Rh-negative women who give birth to a Rh-positive child.
Anti-Rh(D) Immunoglobulin Administration in Pregnancy
Labor plays a crucial role in in many cases of Rh(D) sensitization. Thus, immunoprophylaxis during pregnancy is of fundamental importance. Antenatal anti-Rh(D) immunoglobulin administration can be carried out intramuscularly or intravenously. It may be given once (1,500 IU) at 28–34 weeks of gestation, or twice at 28 and 32–34 weeks of gestation (625 IU or 1,500 IU at both gestational ages).
Miscarriage
Immunoprophylaxis should be given only to women with a spontaneous or medical management of miscarriage after 10 weeks of gestation. In case of a surgical management of miscarriage, it is suggested that prophylaxis may be considered also before 10 weeks of gestation.
Ectopic Pregnancy
Anti-Rh(D) immunoglobulin administration is strongly advised in case of ectopic pregnancy because it has been shown that a ruptured tubal pregnancy is associated with a 24% incidence of alloimmunization to Rh(D).
Chorionic Villus Sampling (CVS) Or Amniocentesis
In most, but not in every, countries it is suggested to administer anti-Rh(D) immunoglobulin to Rh(D)-negative pregnant women after CVS or amniocentesis.
Bleeding and Abdominal Trauma in Pregnancy
Immunoprophylaxis with the administration of anti-Rh(D) immunoglobulin is advised in case of abdominal trauma on the base that this condition may cause fetal–maternal transfusion and consequently an increase in the risk of Rh(D) alloimmunization. The dose of anti-Rh(D) immunoglobulin most commonly administered is 1500 UI.
Intrauterine Fetal Death
As the intrauterine death of a fetus may be the result of a conspicuous fetomaternal hemorrhage (FMH), the amount of the bleeding should be determined with appropriate test, such as the Kleihauer–Betke test, to determine the correct anti-D immunoglobulin dose needed.
External Cephalic Version in the Case of Breech Presentation
The external cephalic version procedure has a risk of FMH ranging from 2 to 6%. Thus, it is advised to administer immunoprophylaxiswith anti-Rh(D) immunoglobulin at 32 week of gestation.
What is non-invasive RhD fetal DNA test?
Fetal Rh(D) status may be determined by non-invasive prenatal testing of cell-free DNA performed in the first trimester of pregnancy. Health policymakers should include this non-invasive testing as a future option for combating Rh disease.
How many doses of anti-D injection do I need?
The dose of anti-Rh(D) immunoglobulin to be given to a Rh-negative pregnant woman varies depending on the different conditions.
High priority
Determine the maternal Rh factor, preferably in early pregnancy.
For Rh(D)-negative women, determine the Rh factor of the newborn from umbilical cord blood. Administer anti-Rh(D) immunoglobulin within 72 hours of delivery to women with a Rh(D)-positive newborn, unless already sensitized.
Use a dose of 500 IU (100 µg) of anti-Rh(D) immunoglobulin; if affordable and with sufficient supply, 1500 IU (300 µg) may be given, as is common in high-income countries. The intramuscular route is as effective as the intravenous route.
Middle priority
Routine anti-Rh(D) prophylaxis during pregnancy: 1500 IU (300 µg) at 28–34 weeks. Anti-Rh(D) immunoglobulin prophylaxis (500 IU; 100 µg) after a surgical abortion or ectopic pregnancy (all gestational ages), or after spontaneous or medical abortion/miscarriage after 10 weeks.
Anti-Rh(D) prophylaxis after bleeding, abdominal trauma in pregnancy, and/or fetal death (500 or 1500 IU; 100 or 300 µg) during the second or third trimester. Kleihauer–Betke test can be used to estimate the optimal dose.
Low priority
Anti-Rh(D) prophylaxis after amniocentesis, chorionic villus sampling, or external cephalic version (500 IU; 100 µg).

